홀덤사이트 : 추천 업체 순위 모음*홀덤 방법 완벽 가이드 – 홀덤메이커
현재 한국에 존재하는 홀덤사이트 업체 중, 국내에서 최초로 서비스하는 실시간 유저 vs 유저 홀덤사이트가 있다는걸 여러분은 아셨나요?
저희 홀덤메이커 홈페이지 에서는 흥미진진하고 스릴이 넘치는 P to P (플레이어 vs 플레이어) 온라인 홀덤사이트 추천을 해드리고 있습니다.
또한 모바일 홀덤사이트, 현금홀덤사이트, 해외 홀덤사이트, 사설홀덤사이트, 온라인홀덤순위 정보를 한눈에 확인 하실 수 있습니다.
홀덤 게임을 즐기는 유저는 필수로 숙지 해야할 홀덤사이트 사용법 완벽 가이드, 홀덤 용어, 홀덤 족보, 7가지 꿀팁 정보 제공을 하고 있으니 꼭 확인 해보세요.

홀덤사이트 :: 추천 업체 순위
국내에 존재하는 많은 온라인 홀덤사이트 업체들 중, 가장 안전한 메이저 홀덤사이트 업체를 엄격하게 선별하여 소개 해드립니다.
저희 홀덤메이커 에서 추천 해드리는 홀덤사이트는 높은 자본금을 바탕으로 튼튼하게 운영하고 있으며, 신속하고 정확한 고객 응대를 자랑 합니다.
그리고 제일 중요한 입금 및 출금 관련 하여 오랫동안 사고 없는 무사고 홀덤사이트 업체만 선별하여 추천 해드립니다.
홀덤사이트 자주 묻는 질문
- 홀덤사이트 개요 뜻
- 홀덤사이트 소개
- 홀덤사이트 게임 진행 방법
- 홀덤 용어
- 홀덤 족보
- 홀덤 7가지 필수 팁
- 홀덤사이트 단독 이벤트
1. 홀덤사이트의 개요 뜻
홀덤사이트 개요 뜻 ?
홀덤은 포커 카드로 진행 되는 게임 입니다.
오프라인에서 여럿 사람들과 모여서 홀덤 게임을 진행 하기란 결코 쉽지 않습니다.
특성상 제재도 심하며, 불특정 다수의 사람들과 모여서 홀덤 게임을 진행 하는 장소를 찾기란 쉽지 않으며, 안전 또한 보장을 할 수 없습니다.
그래서 생겨난 것이 바로 온라인 홀덤사이트 입니다. 실제로 만날 필요 없이, 온라인 상에서 진행되는 홀덤사이트는 24시간 언제든지 편리하게 접속하여 불특정 다수의 사람들과 재밌고 즐겁게 홀덤을 즐기실 수 있습니다.

2. 홀덤사이트 소개
홀덤사이트 소개 : 앞서 설명 해드렸던 내용에 추가로, 저희 홀덤메이커 홈페이지 에서는 국내 최초 컨텐츠 실시간 유저 vs 유저 (유저 대 유저)
온라인 홀덤사이트 소개 및 추천을 해드리고 있습니다.
하지만 이용 도중 문제 또는 사고가 생기면 절대 안되겠죠? 오랫동안 회원들에게 인정받고 사고없는 안정적인 업체만 선별하여 추천 해드리고 있습니다.
홀덤사이트를 이용 하시려는 고객 분들께서는 걱정 끝! 안심하시고 사용 하시면 됩니다.
홀덤사이트 추천 순위 모음 게시판에서 안전한 업체를 만나 보세요.

3. 홀덤사이트 게임 진행 방법
홀덤사이트 게임 진행 방법 : 홀덤은 52장의 포커 카드로 플레이 합니다.
한 테이블에 최소 2명~ 최대9명의 플레이어 유저가 게임을 플레이 합니다.
최대 6명이 앉는 테이블은 6링, 최대 9명이 앉는 테이블은 9링 이라고 말 합니다.
게임이 진행 방법은 딜러가 각각의 플레이어 유저들에게 카드 두 장씩 카드를 분배 합니다.
그리고 테이블 중앙 바닥에 5개의 카드를 오픈 합니다. (명칭 : 커뮤니티 카드)
커뮤니티 카드와 플레이어 유저 에게 지급한 2개의 카드를 조합하여 가장 높은 족보를 만들어서 족보가 가장 높은 사람이 승리 합니다.
한국에서 유명한 영화 “타짜” 에서 나오는 섯다 해외 버전이라고 생각하시면 이해 하시기 매우 쉽습니다.
더 자세한 홀덤 가이드는 홀덤 방법 완벽 가이드 게시판에서 자세히 확인 하실 수 있습니다.

4. 홀덤 용어
홀덤 용어 는 간단하게 몇가지 단어만 숙지해도 게임을 진행 하는데 큰 무리가 없습니다.
홀덤 자체가 국제적인 게임 이므로, 용어가 대부분 영어 이기 때문에 몇가지 단어는 외우시는게 게임을 진행할때 이해 하기가 좋습니다.
하지만 너무 걱정 안하셔도 됩니다, 몇판 하다보면 금새 익숙해 지기 때문에 대략 이런 단어가 있다 라는 것만 숙지 하셔도 좋습니다.
● 프리플랍 : 각 플레이어 유저가 2장의 카드를 받은 상태
● 플랍 : 테이블 중앙 바닥에 3장의 커뮤니티 카드 오픈 상태
● 턴 : 테이블 중앙 바닥에 4번째의 커뮤니티 카드 오픈
● 리버 : 테이블 중앙 바닥에 마지막 5번째 커뮤니티 카드 오픈
● 블러프 : 플레이어 유저가 실제 갖고 있는 카드가 낮은 족보이지만 높은 족보인것 처럼 속이는 행위.
● 키커 : 배팅의 순서가 전부 진행되고 마지막에 카드를 오픈했을때 최종 5개의 카드 족보가 상대방과 같을 경우 승부를 마무리 짓는 결정적인 마지막 1장의 카드.
● 쇼다운 : 각 플레이어 유저의 카드 패를 오픈하여 승부를 결정 짓는 용어
● 체크 : 본인 차례에서 판돈을 배팅 하지 않고 다음 사람에게 턴을 넘기는 용어
● 벳 : 본인이 가장 먼저 판돈을 배팅 하는 용어
● 레이스 : 바로 직전 플레이어 유저의 배팅 금액에서 판돈을 더 배팅하는 용어
● 폴드 : 게임을 포기 하는 용어
간단하게 이정도 용어가 있습니다. 하지만 홀덤 용어는 엄청나게 더 많은거 알고 계신가요?
홀덤 용어 게시판에서 더 자세한 정보를 한 눈에 확인 하실 수 있습니다.

5. 홀덤 족보
홀덤 족보 높은 순서대로 나열 입니다.
홀덤사이트 사용 하기 전, 플레이어 유저 에게 꼭 필요한 필수 정보 입니다.
● 로얄 플러시 : 그림과 색깔이 같은 A, K, Q, J, 10 카드 이며 가장 높은 족보 입니다.
● 스트레이트 플러시 : 그림과 색깔이 같으며 연속으로 나열되는 숫자 카드 입니다.
● 포 카드 : 동일한 숫자 또는 영어 카드 4장으로 이루어진 족보 입니다. 나머지 카드 1장으로 승자를 정합니다.
● 풀 하우스 : 동일한 숫자 카드 3장 과 동일한 숫자 카드 2장 으로 이루어진 족보 입니다.
● 플러시 : 숫자에 상관 없이 카드 5장의 무늬가 같은 족보 입니다.
● 스트레이트 : 무늬에 상관 없이 숫자가 연속으로 나열된 족보 입니다.
● 트리플 : 동일한 숫자 또는 영어 3장의 족보 입니다.
● 투페어 : 동일한 숫자 2장 2장, 쌍의 족보 입니다.
● 원페어 : 동일한 숫자 2장 1쌍의 족보 입니다.
● 하이카드 : 족보에 없는 카드 조합 입니다. 카드 중 제일 높은 숫자로 승자를 정합니다.
홀덤 족보에 대하여 더 자세한 이미지와 설명은 홀덤 족보 게시판에서 한 눈에 확인 가능 합니다.
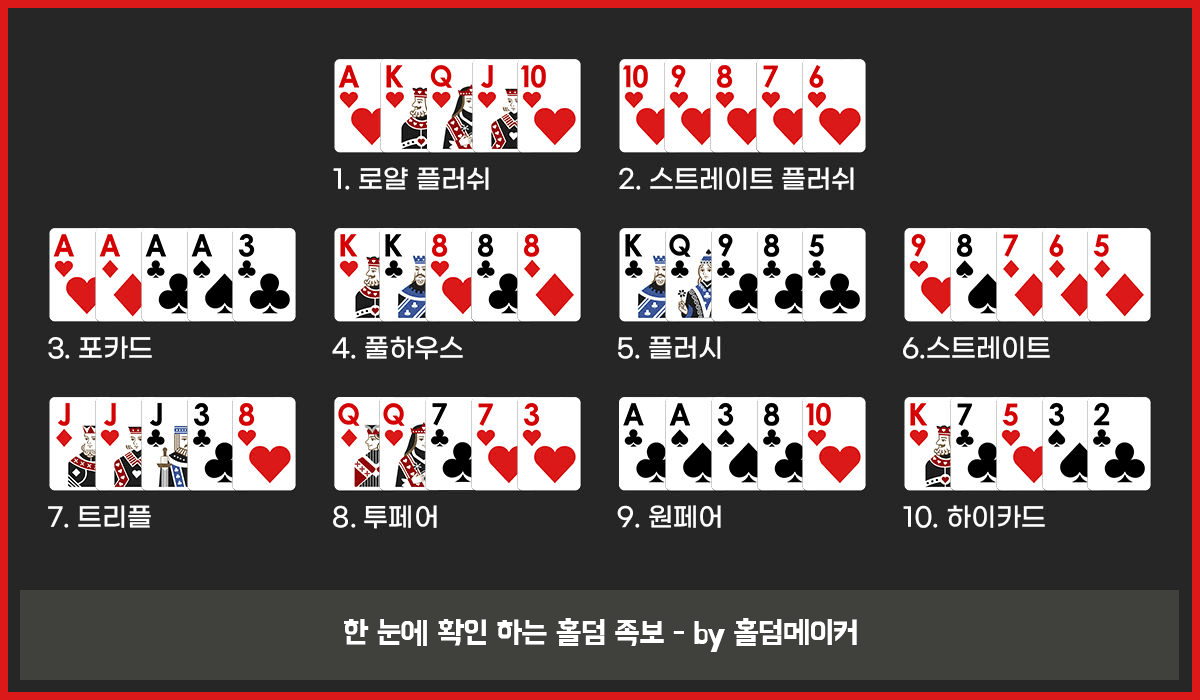
홀덤 족보 에서 가장 높은 조합은 로얄 플러시 입니다.
게임 시작 후 처음 카드 2장을 지급 받았을때, 가장 좋은 핸드 카드는 A,A 입니다.
반대로 제일 안좋은 핸드 카드는 서로 다른 그림의 2,7 입니다.

6. 홀덤 7가지 필수 팁
홀덤메이커 에서 무료로 제공하는 전문적인 가이드 정보 홀덤 7가지 필수 팁 내용 입니다.
홀덤은 몇판 하다보면 금방 배울수 있습니다. 하지만 홀덤메이커 에서 무료로 제공하는 전문적인 지식과 꿀팁 정보를 숙지 하신다면
고객 여러분들 승리를 하는데 도움이 될 수 있을거라 자신 있게 말씀 드릴 수 있습니다.
● 처음 받는 2장의 핸드 카드를 주목 하시기 바랍니다.
홀덤을 진행하는 유저의 대부분은 첫 카드 2장이 추후에 어떤 조합으로 이루어 지는지 정확히 파악을 해야 합니다.
내가 좋은 카드를 받았는지, 나쁜 카드를 받았는지 빠르게 판단하여 폴드를 하거나, 레이스를 하는 것이 매우 현명한 선택 입니다.
● 순서 포지션에 따른 전략
나의 순서가 처음이냐 마지막이냐 에 따라서 해당 판의 분위기를 미리 확인 할 수 있습니다.
만약 내가 마지막 순서 포지션 이라면, 나의 차례가 오기 전 까지 다른 플레이어 유저들이 어떤식으로 배팅을 하는지 확인 하여 맞대응 하시기 바랍니다.
몇판 하면서 순서가 돌아가다 보면, 아~ 저 사람이 지금 구라를 치고 있구나, 아니면 강력한 패를 들고 있구나 라는 것을 파악 할 수 있습니다.
● 카드 조합 족보 빠른 판단
나의 순서가 몇 차례 오기 전 까지 스트레이트 또는 플러시를 완료 할 수 없다면, 과감히 폴드 해서 카드를 버려야 합니다.
무리한 레이스와 기대감은 패배로 가는 지름길 입니다.
● 블러프
블러프는 나의 카드 조합이 더 강력하다고 믿게 만들어서, 상대방을 제압하여 폴드 시키는 방법 입니다.
강력한 족보로 승리 하는 방법이 제일 확실 하지만, 때로는 블러프를 사용하여 상대방에게 혼란을 주어 승리를 하는 짜릿한 스킬도 꼭 갖추어야 할 하나의 스킬 입니다.
● 타이밍 체크
내가 만약 한 곳에서 계속 “다운스윙” 즉 계속 패배를 하고 있다면 그 자리를 떠나는 것은 매우 어렵습니다.
이것은 사람의 본능과 직결 되는데 승부욕 또는 나의 재산을 잃게 되어서 본전 또는 그 이상을 회수 하기 위한 본능 때문 입니다.
하지만 진정한 고수가 되려면 다운스윙이 계속 되면 미련없이 그 판을 떠나야 합니다.
마인드 컨트롤을 다시 셋팅 하여, 새로운 마음가짐으로 다른 플레이어 유저 들이 있는 곳으로 이동 하는 것도 하나의 스킬 전략 입니다.
● 칩으로 공격하기
“노 리밋” 자신이 갖고 있는 칩 범위 내에서 일부 또는 전부 배팅을 할 수 있는 규칙 입니다.
이 규칙을 이용 하여 상대방에게 무리한 베팅을 강요 하거나 도발 할 수 있습니다.
이 전략은 강력한 족보를 갖고 있지 않는 이상 상대방을 폴드 시켜서 포기 하게 만드는 꿀팁 입니다.
● 전략적인 레이스
9명의 플레이어 유저가 같은 방에서 지속적으로 홀덤을 진행 하다보면, 특징이 뚜렷하게 보이는 플레이어 유저가 생겨 날 것 입니다.
예를 들면 계속 폴드 하다가 갑자기 크게 레이스를 하는 동일한 진행 방식의 플레이어는, 다른 플레이어 유저가 봤을때 무조건 높은 족보 라고 생각하여
대부분 폴드를 하기 때문에, 해당 유저는 높은 족보를 손에 쥐어도 큰 금액을 가져가기 힘들 것 입니다.
본인 에게 높은 족보의 카드가 들어 왔더라도, 포커페이스를 유지하여 조금씩 레이스 하는 습관을 가지시기 바랍니다.

7. 홀덤사이트 단독 이벤트
홀덤사이트 단독 이벤트 진행!
홀덤메이커에서 소개 해드리는 업체는 신규 첫 가입 첫 충전시 높은 요율로 현금처럼 사용 가능한 보너스 금액을 지급 해드리고 있습니다.
또한 매번 게임을 진행 할 때마다 쌓이는 롤링 콤프 보너스를 지급하는 업체도 있습니다.
당일 첫 충전시 보너스를 지급 하거나, 올인 위로금 보너스 지급, 생일 쿠폰, 출석체크 이벤트 등
오직 홀덤메이커 홈페이지 에서 받을수 있는 이벤트 혜택을 놓치지 말고 꼭 챙기시기 바랍니다.
해당 이벤트는 각 업체 마다 조금씩 차이가 있음을 알려 드립니다.

홀덤사이트 정보는 홀덤메이커 입니다
홀덤사이트의 모든 정보 및 사용법 가이드 이벤트 혜택, 홀덤에 대한 모든 정보가 있는 곳 홀덤 메이커 입니다





